 Atlanta, GA – Influenza causes considerable morbidity and mortality in the United States. Between 2010 and 2020, an estimated 9–41 million cases resulted in 140,000–710,000 hospitalizations and 12,000–52,000 deaths annually (1).
Atlanta, GA – Influenza causes considerable morbidity and mortality in the United States. Between 2010 and 2020, an estimated 9–41 million cases resulted in 140,000–710,000 hospitalizations and 12,000–52,000 deaths annually (1).
As the United States enters the 2021–22 influenza season, the potential impact of influenza illnesses is of concern given that influenza season will again coincide with the ongoing COVID-19 Coronavirus pandemic, which could further strain overburdened health care systems.
The data analyzed were reported from 11 U.S. jurisdictions with high-performing state immunization information systems.* Overall, influenza vaccine administration was 9.0% higher in 2020 compared with the average in 2018 and 2019, combined.
However, in 2020, the number of influenza vaccine doses administered to children aged 6–23 months and children aged 2–4 years, was 13.9% and 11.9% lower, respectively than the average for each age group in 2018 and 2019. Strategic efforts are needed to ensure high influenza vaccination coverage among all age groups, especially children aged 6 months–4 years who are not yet eligible to receive a COVID-19 vaccine.
Summary
What is already known about this topic?
As the United States enters the 2021–22 influenza season, influenza-associated morbidity and mortality could further strain health care systems already overburdened by the ongoing COVID-19 pandemic.
What is added by this report?
During September–December 2020, overall influenza vaccine administration was 9.0% higher than the average during September–December in 2018 and 2019; however, the number of administered doses declined among children aged 6–23 months (13.9%) and 2–4 years (11.9%).
What are the implications for public health practice?
Continued strategic efforts are needed to ensure high influenza vaccination coverage among all eligible persons aged ?6 months, especially children aged ?4 years.
Administration of influenza vaccine and a COVID-19 vaccine among eligible populations is especially important to reduce the potential strain that influenza and COVID-19 cases could place on health care systems already overburdened by COVID-19.
Influenza vaccination data reported to CDC from 11 study jurisdictions† with high-performing state immunization information systems for persons in the following age groups were analyzed: 6–23 months, and 2–4, 5–12, 13–17, 18–49, 50–64, and ?65 years.
[470cetner]
Persons aged ?6 months with at least 1 dose of influenza vaccine administered between the first week of September and last week of December in 2018, 2019, and 2020, were included in the analysis. The numbers of vaccine doses administered to each age group in 2020 were compared with the average number of reported doses administered during the corresponding weeks in 2018 and 2019.
In addition, the percentage change between the number of influenza vaccine doses administered during September–December 2020 and the average administered in the corresponding periods in 2018 and 2019 among persons aged ?6 months was calculated overall and stratified by age groups.

Analyses were conducted with SAS (version 9.4; SAS Institute). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.§
A total of 16,872,970 influenza vaccine doses were reported by 11 study jurisdictions to state immunization information systems during September–December 2020, compared with an average of 15,513,428 doses reported during the same weeks in 2018 and 2019 (Figure 1), representing an overall increase of 9.0% in influenza doses administered to all age groups compared with 2018 and 2019 (Figure 2).
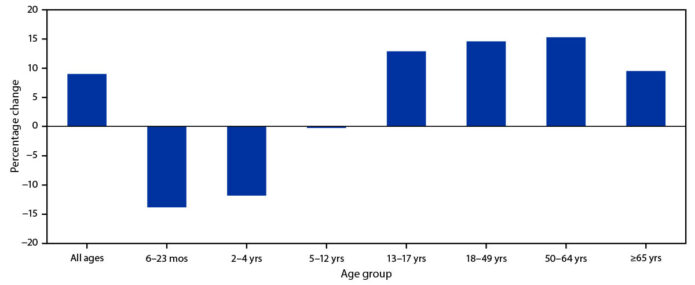
However, the numbers of influenza vaccine doses administered to children aged 6–24 months and children aged 2–4 years were 13.9% and 11.9% lower, respectively than the average numbers administered during September–December of 2018 and 2019. The number of doses administered to children aged 5–12 years was similar in 2018, 2019, and 2020.
During September–December 2020, the number of influenza vaccine doses administered increased 12.9% among adolescents aged 13–17 years, the only increase observed among all children, compared with the average during the corresponding period in 2018 and 2019. Influenza doses administered to adults increased in all age groups during September–December 2020, compared with the average during the preceding 2 years: the largest increase (15.3%) was among persons aged 50–64 years, followed by persons aged 18–49 years (14.6%); the smallest increase was among persons aged ?65 years (9.5%).
1Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 2CDC COVID-19 Rapid Response Team; 3Peraton Corporation, Herndon, Virginia; 4Stat-Epi Associates, Inc, Ponte Vedra Beach, Florida; 5Idaho Department of Health and Welfare; 6Iowa Department of Public Health; 7Louisiana Department of Health; 8Michigan Department of Health and Human Services; 9Minnesota Department of Health; 10New York City Department of Health and Mental Hygiene, New York; 11North Dakota Department of Health; 12Oregon Health Authority; 13Utah Department of Health; 14Washington State Department of Health;15Wisconsin Department of Health Services.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* A high-performing immunization information system was defined as a system with vaccine estimates within 10 percentage points of those from the 2018 National Immunization Survey-Child and National Immunization Survey-Teen (https://www.cdc.gov/vaccines/imz-managers/nis/about.html), and which recorded ?90% of doses administered to persons aged <19 years that were submitted and processed within 30 days of vaccine administration.
† Study jurisdictions included Idaho; Iowa; Louisiana; Michigan; Minnesota; New York, New York; North Dakota; Oregon; Utah; Washington; and Wisconsin. Immunization information systems are confidential, computerized, population-based systems that collect and consolidate vaccination data from providers in 64 jurisdictions nationwide and can be used to track administered vaccines and measure vaccination coverage. The 64 jurisdictions include the 50 U.S. states, five U.S. territories (American Samoa, Guam, Northern Mariana Islands, Puerto Rico, and U.S. Virgin Islands), three freely associated states (Federated States of Micronesia, Marshall Islands, and Palau), and six local jurisdictions (Chicago, Illinois; Houston, Texas; New York, New York; Philadelphia, Pennsylvania; San Antonio, Texas; and Washington, DC).
§ 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.



