Risk of Poisoning
 Washington, D.C. – The U.S. Consumer Product Safety Commission, in cooperation with the firm named below, today announced a voluntary recall of the following consumer product. Consumers should stop using recalled products immediately unless otherwise instructed.
Washington, D.C. – The U.S. Consumer Product Safety Commission, in cooperation with the firm named below, today announced a voluntary recall of the following consumer product. Consumers should stop using recalled products immediately unless otherwise instructed.
Triaminic and Theraflu Products are being recalled because the child-resistant caps can fail to work properly which enables the cap to be removed by a child posing a risk of unintentional ingestion and poisoning.
Consumers should stop using this product unless otherwise instructed. It is illegal to resell or attempt to resell a recalled consumer product.
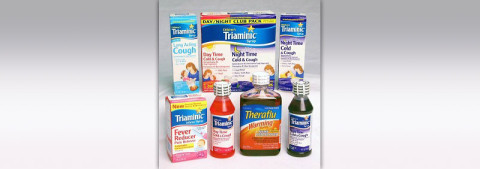
Product Information
Name of product: Triaminic® Syrups and Theraflu Warming Relief® Syrups
Manufacturer: Novartis Consumer Health Inc., of Parsippany, NJ
Units: About 2.3 Million
Hazard: These child-resistant caps can fail to function properly and enable the cap to be removed by a child with the tamper-evident seal in place, posing a risk of unintentional ingestion and poisoning. These products contain acetaminophen and diphenhydramine which are required by the Poison Prevention Packaging Act to be sealed with child-resistant packaging.
Incidents/Injuries: The firm has received 12 reports of children unscrewing the locked caps, including four reports of children ingesting the product. One child required medical attention.
Description: This recall involves Triaminic® Syrups and Theraflu Warming Relief® Syrups for coughs, colds and fevers. There are 24 types of these two products included in the recall. A complete list of products, lot numbers and National Drug Codes (NDC) can be found at www.novartisOTC.com. Lot numbers are located on the bottom panel of the box and on the left side of the label on the bottle. The NDC number is located on the upper right corner of the front panel of the Triaminic Syrups box and the upper left corner of the Theraflu Warming Relief Syrups bottle.
Sold at: Food, drug, mass merchandise and club stores nationwide between May 2010 and December 2011 for about $5.00.
Manufactured in: U.S.A.
Remedy: Consumers should immediately stop using the recalled product and contact Novartis for instructions on how to return the product for a full refund.
Consumer Contact: Novartis Consumer Healthcare toll-free at 866.553.6742 from 8:00am to midnight ET, Monday through Saturday, or online at www.novartisOTC.com for more information.
The U.S. Consumer Product Safety Commission (CPSC) is still interested in receiving incident or injury reports that are either directly related to this product recall or involve a different hazard with the same product. Please tell us about your experience with the product on SaferProducts.gov
About the U.S. Consumer Product Safety Commission
CPSC is charged with protecting the public from unreasonable risks of injury or death associated with the use of the thousands of consumer products under the agency’s jurisdiction. Deaths, injuries, and property damage from consumer product incidents cost the nation more than $900 billion annually. CPSC is committed to protecting consumers and families from products that pose a fire, electrical, chemical, or mechanical hazard. CPSC’s work to ensure the safety of consumer products – such as toys, cribs, power tools, cigarette lighters, and household chemicals – contributed to a decline in the rate of deaths and injuries associated with consumer products over the past 30 years.
Under federal law, it is illegal to attempt to sell or re-sell a recalled product.
To report a dangerous product or a product-related injury, go online to: SaferProducts.gov, call CPSC’s Hotline at 800.638.2772 or teletypewriter at 301.595.7054 for the hearing and speech impaired. Consumers can obtain this news release and product safety information at www.cpsc.gov.


